McMaster’s Inhaled COVID-19 Vaccine Enters Phase 2 Trials
In a move that raises significant concerns, researchers at McMaster University have launched Phase 2 clinical trials for a next-generation inhaled COVID-19 vaccine. Dubbed the AeroVax study, this trial is backed by $8 million in funding from the Canadian Institutes of Health Research (CIHR) and aims to test a needle-free alternative designed to provide enhanced protection against SARS-CoV-2.
The Experiment Continues
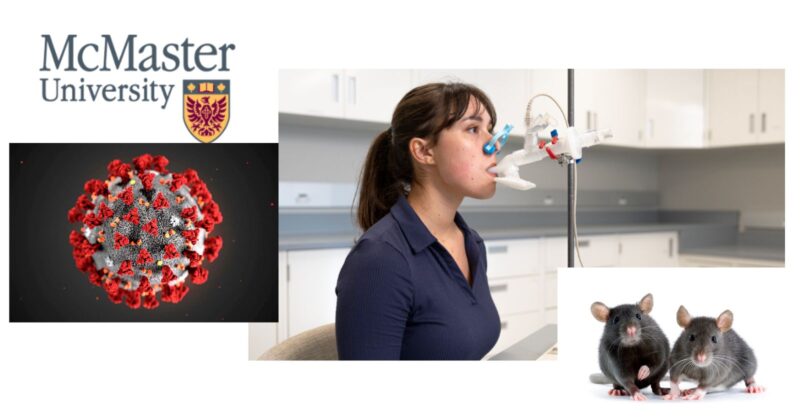
Despite widespread controversy surrounding COVID-19 vaccines, McMaster researchers Fiona Smaill and Zhou Xing are pressing forward with a multicentre trial to evaluate both the safety and effectiveness of this novel inhaled vaccine. Unlike traditional injections, this vaccine directly targets the lungs and upper airways—the virus’s initial point of entry. Early findings claim that this approach generates a stronger immune response.
Professor Smaill acknowledges that existing COVID-19 vaccines have not prevented recurrent infections but insists that this new formulation could change that. However, given the numerous concerns regarding past vaccine safety, is this yet another rushed attempt at medical intervention?
Canadian-Made, But At What Cost?
This inhaled vaccine has been developed entirely in Canada, from its design and biomanufacturing at McMaster’s Robert E. Fitzhenry Vector Laboratory to its clinical testing at various Canadian research sites. The Phase 2 trial will enroll 350 participants in Hamilton, Ottawa and Halifax, with strict eligibility criteria:
- Must have received at least three doses of an mRNA COVID-19 vaccine
- Must not have received AstraZeneca’s COVID-19 vaccine
- No prior COVID-19 infection or vaccination within three months
- No lung disease diagnosis
- Must be between 18 and 65 years old
- Must be available for in-person visits
This randomized, placebo-controlled trial will see two-thirds of participants receive the inhaled vaccine while the remaining third gets a placebo. Researchers claim this process ensures safety and effectiveness, yet concerns about transparency, long-term effects, and rushed approvals linger.
What Comes Next?
Following Phase 2, McMaster researchers plan to push forward with Phase 3 trials, expanding testing to a larger population with the goal of securing market approval. Given past issues with COVID-19 vaccine mandates, adverse reactions and the shifting scientific narratives, the question remains: why would anyone trust yet another experimental vaccine?
Story & Participant Photo Source: McMaster University
Here’s the story of a COVID-19 vaccine trial participant…once Brianne Dressen was injured, AstraZeneca removed her from the trial and never spoke to her again.
********************************************************************************************************************************************
If you find value in the work we do at Children’s Health Defense Canada, please consider making a donation.