Major MMR Death Signal Analysis Raises New Safety Questions
Safety Signal Findings Rekindle Questions Regulators Can No Longer Ignore
A newly published safety signal analysis is drawing renewed attention to deaths reported following measles, mumps, and rubella (MMR) and MMRV vaccination in the United States, intensifying debate over vaccine safety surveillance and regulatory transparency.
The study examines deaths reported to the U.S. Vaccine Adverse Event Reporting System (VAERS), a passive surveillance database designed to detect potential adverse event patterns following vaccination.
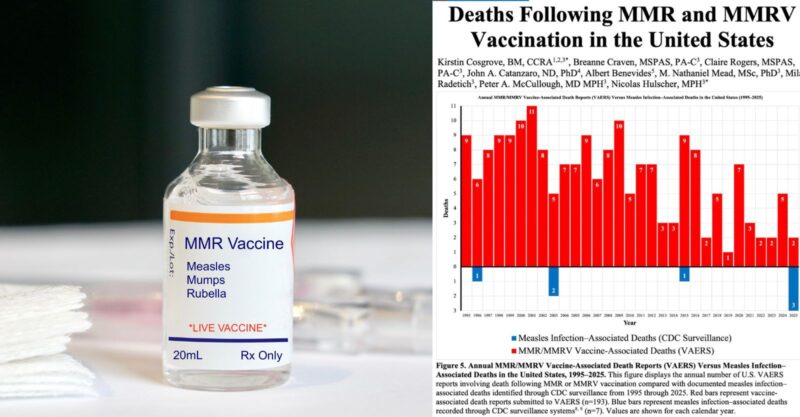
According to the McCullough Foundation study, researchers identified what they describe as a mortality safety signal associated with MMR and MMRV products. The analysis reports that a majority of deaths occurred in very young children, with 60.9% involving those under the age of two. A substantial proportion of reported deaths were described as occurring shortly after vaccination, with approximately 40% taking place within one week of injection and most clustering within a two-week period.
The study further notes that nearly one quarter of reported deaths were categorized as Sudden Infant Death Syndrome (SIDS). Frequently cited clinical events included cardiac arrest, seizures and encephalitis.
In addition to examining temporal patterns, the authors compared cumulative measles infection deaths recorded since 1995 with deaths reported following vaccination, calculating what they characterize as a “2,657% higher death count.”
Signal Detection vs. Causation
VAERS functions as an early warning system rather than a mechanism for determining causality. Reports submitted to the database do not establish that a vaccine caused a particular event, and entries may include incomplete information, coincidental outcomes, or events requiring further verification.
Regulatory authorities have consistently emphasized that safety signals detected through passive surveillance systems must be interpreted cautiously and investigated using controlled epidemiological studies. At the same time, critics argue that passive reporting systems may under capture adverse events.
Research frequently cited in this context has suggested that adverse event reporting rates may be incomplete, contributing to ongoing disagreement over how safety signals should be evaluated and communicated.
Renewed Debate Over Surveillance Transparency
The publication of the analysis has rekindled broader discussion surrounding vaccine safety monitoring, including how signals are assessed, how quickly investigations are initiated, and how findings are disclosed to the public.
Advocates for greater transparency argue that safety signals involving pediatric outcomes warrant prompt and independent examination. Public health authorities, meanwhile, stress the importance of distinguishing between correlation and causation when interpreting passive surveillance data.
Relevance for Canadian Readers
Although based on U.S. reporting data, the issues raised resonate in Canada, where debates over vaccine safety surveillance, regulatory transparency, and informed consent continue.
Safety signal analyses — especially those involving childhood vaccines — provoke strong reactions. Yet public confidence depends on transparent safety data, honest acknowledgment of limitations, and independent scientific scrutiny.
The central question remains whether emerging safety signals will be openly and rigorously examined.
Conclusion
For too many families, this is lived reality.
Parents who report that a child was healthy before the MMR/MMRV and gone soon after are too often dismissed. Yet safety surveillance systems exist precisely because potential harms must be detected, examined and transparently investigated.
Sources:
Zenodo Study Repository — Deaths Following MMR and MMRV Vaccination in the United States
Nicolas Hulscher, MPH, The Focal Points Substack — “BREAKING STUDY: MMR and MMRV Vaccines Linked to 2,657% More U.S. Deaths Than Measles Infection Since 1995”
U.S. Vaccine Adverse Event Reporting System (VAERS)
Centers for Disease Control and Prevention (CDC) – Measles Data
Our study of CDC data found that the MMR and MMRV shots are linked to 2,657% more American deaths than measles infection since 1995.
For the past 30 YEARS, the vaccine has been FAR more deadly than the disease — inducing SIDS and cardiac arrests among our children. https://t.co/GmdwFuU7hp pic.twitter.com/mf1QGdVv81
— Nicolas Hulscher, MPH (@NicHulscher) February 18, 2026
The Science of the MMR Vaccine – MMRII
Priorix MMR
ProQuad MMRV
The troubling history of the MMR vaccine
******************************************************************************************************************