Changes in Japan’s Infant Vaccination Schedule and SIDS Trends (1970s–1980s)
DTP Vaccine Raised Deadly Vaccine Concerns in 1970s Japan leading to a pause and a delay in their use. AMA, APA, Pediatricians need to remember that trust depends on transparency, not paternalism.
Here are the facts as can best be found in the scientific record. The exonerating studies on pertussis vaccines and SIDS have problems.
DTP Raised Deadly Vaccine Concerns in 1970s Japan
Timeline of Key Vaccine Policy Changes (1975–1989)
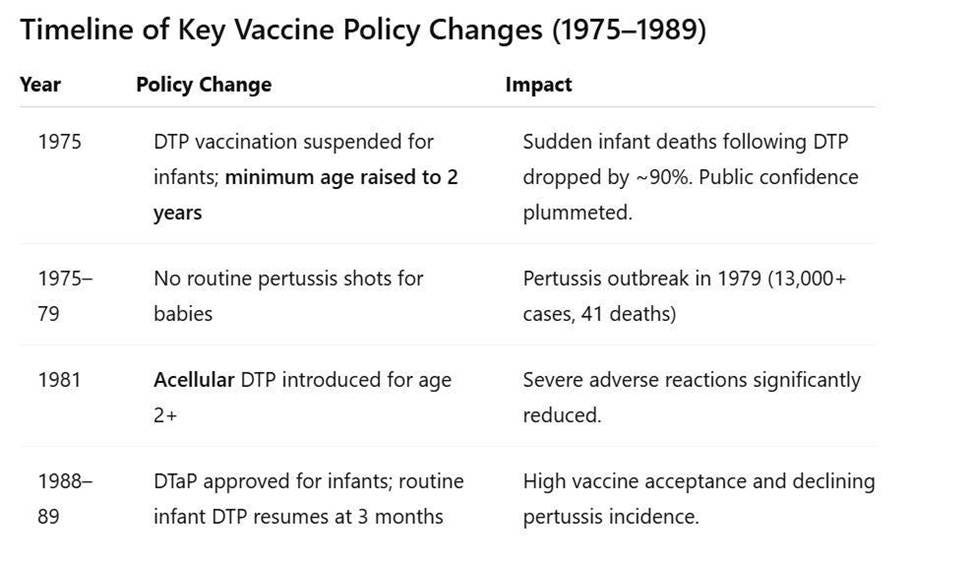
In 1975, DTP vaccination was suspended for infants. After infant deaths were linked temporally to DTP, the government temporarily halted pertussis immunizations. The program was revamped to delay DTP until age 2 (instead of infancy). As a direct result, reports of sudden deaths following vaccination virtually disappeared in the ensuing years. In fact, vaccine injury compensation for post- DTP sudden deaths dropped from 37 cases (1970–1974) to only 3 cases in the period 1975–1981. According to an American Academy of Pediatrics task force, the category of “sudden death” as a vaccine-related report “disappeared…when immunization was delayed until a child was 24 months of age” (Cherry et al., 1988). This delay also markedly reduced other severe adverse reactions temporally associated with DTP in infants.
(It is worth noting that Cherry et al. (1988) is a secondary analysis conducted by a U.S.-based AAP task force and not based on original Japanese health system records. Its interpretation reflects Western diagnostic conventions and may not fully represent Japanese reporting practices of the time.)
By the late 1970s, Japan saw a pertussis resurgence. The curtailment of infant DTP led to a steep decline in pertussis vaccination coverage. Consequently, whooping cough (pertussis) cases surged. In the three years before the 1975 suspension, Japan had only about 400 pertussis cases and 10 deaths; in the three years after stopping infant shots, cases spiked to over 13,000 with 113 deaths. A major nationwide pertussis outbreak in 1979 infected tens of thousands; 41 infant deaths were attributed to pertussis. This tragedy underscored the vaccine’s benefits – a point recognized by the public and government in hindsight.
In 1981, the acellular pertussis vaccine (DTaP) was introduced. In response to safety concerns, Japan became the first country to develop and approve an acellular pertussis (aP) vaccine – a safer formulation containing purified pertussis components instead of whole bacterial cells. Starting late 1981, acellular DTP (often called DTaP) completely replaced the old whole-cell DTP for routine use. Initially, the acellular vaccine was given only to children age 2 years, continuing the policy of delayed start. From 1982–1988, over 40 million doses of acellular pertussis vaccine were administered to Japanese 2-year-olds (Watanabe & Nagai, 2005). Studies confirmed that serious adverse events, including encephalopathy, convulsions, and sudden deaths, were significantly lower with the new acellular DTP compared to the reactogenic whole-cell vaccine (Kuno‐Sakai & Kimura, 2004).
However, acellular pertussis vaccines are also known to reduce symptomatic disease without preventing colonization or transmission. As such, post-vaccine case counts likely underestimated the true burden of circulating Bordetella pertussis, especially among asymptomatic carriers. It is worth noting that because aP vaccination is known to mask infection, and ‘cases’ in the vaccinated exclude asymptomatic infected (Warfel et al., 2013). This is especially problematic because only symptomatic individuals – including healthcare workers- will seek treatment (antibiotics) and self-quarantine to avoid making infants, the most vulnerable population, sick.
In the late 1980s (1988–1989), Japan resumed infant immunization. As confidence in the acellular DTP grew, Japanese health authorities began to move the pertussis vaccine back into infancy. In 1988 the Ministry of Health approved DTaP for infants, and by 1989 many areas of Japan re-introduced the pertussis shot at 3 months of age as part of the routine schedule. Thus, after roughly 14 years of delayed vaccination policy, Japan returned to vaccinating infants in early life – but now with DTaP. High vaccine acceptance was achieved, and pertussis incidence sharply declined again by the 1990s. (For reference, in 1994 Japan formally amended its Immunization Law to end mandatory vaccination, but it continued strong recommendations for all infant vaccines, including DTaP, under age 2.)
SIDS Rates Before, During, and After the Changes
Sudden Infant Death Syndrome (SIDS) – the unexplained sudden death of an apparently healthy infant – was relatively uncommon in Japan during the 1970s and 1980s, especially compared to Western nations. It is important to note that diagnostic practices differed: Japanese authorities often classified unexplained infant deaths under categories like “unknown cause” or suffocation, meaning not all such deaths were formally labeled as SIDS (Sawaguchi et al., 2000). Nonetheless, available data suggest some notable trends around the time of the vaccination policy changes:
Early 1970s (pre-change): With DTP given at 3 months, there were documented cases of infants dying suddenly soon after vaccination. In 1970–1974, Japan recorded 37 infant “sudden deaths” temporally associated with pertussis shots (about 1.47 deaths per million DTP doses). These cases likely would be classified as SIDS or vaccine-related SIDS by today’s standards. They represented a small fraction of overall infant deaths, but their clustering shortly post-vaccination raised national concern.
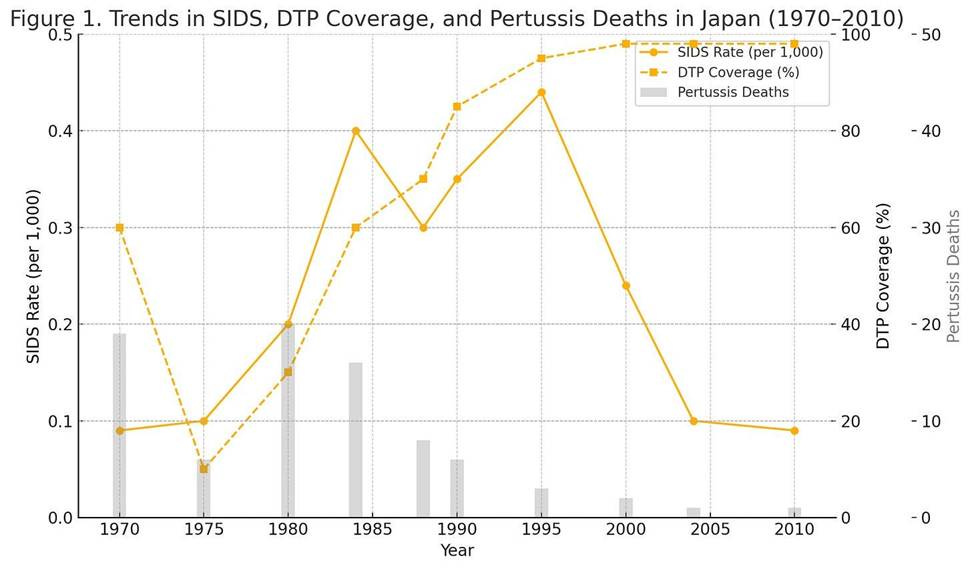
Late 1970s to mid-1980s (during delay period): After 1975, with no infants being immunized against pertussis in their first two years, reports of sudden deaths following vaccination virtually vanished. Japan’s vaccine injury data show that post-immunization sudden death cases fell by ~90% once the DTP series was deferred to age 2 . Indeed, James Cherry’s review noted that “‘sudden death’…disappeared” as a reported phenomenon when infant DTP was removed. In epidemiologic terms, Japan’s SIDS rate during the late 1970s and 1980s remained very low, on the order of only a few tenths of a death per thousand births (Müller-Nordhorn et al., 2020). One international analysis found that unlike many Western countries that saw a mid-1980s peak in SIDS, Japan’s recorded SIDS mortality stayed low throughout the 1980s. For example, by the mid-1980s (1984), Japan’s SIDS rate was roughly 0.4 per 1,000 live births – well below rates in the U.S. or Europe at that time. This low incidence coincided with the period when babies were not receiving DTP in early infancy. Some have interpreted this as a mere correlation: i.e. that delayed vaccination appeared to contribute to lower SIDS occurrence.
Japan began vaccinating infants again by 1989, and through the early 1990s the recorded SIDS rate did increase somewhat. SIDS reached an observed high around the mid-1990s (approximately 0.44 per 1,000 in 1995). Notably, this time period was a peak for SIDS in many countries and also when Japan first aligned with Western-style infant sleep practices. After 1995, however, Japan’s SIDS incidence declined sharply, dropping to about 0.24 per 1,000 by 2001. By the 2000s, SIDS (as an official category) was exceedingly rare in Japan (roughly 0.1 per 1,000, or 0.01%). The halving of SIDS rates from the mid-1980s to early 2000s was largely credited to public health interventions – especially a nationwide “Back-to-Sleep” campaign in the late 1990s that encouraged non-prone sleeping and raised awareness of SIDS risk factors. In short, Japan’s continued long-term decline in infant sudden deaths after the 1980s is attributed more to safe sleep practices and other risk reductions than to vaccination policy.
It is difficult to say with certainty how much the 1975–1988 vaccine delay policy affected Japan’s overall SIDS statistics. The temporal association is more than intriguing: during the years when virtually no infants received DTP, SIDS diagnoses were extremely infrequent. However, absolute SIDS numbers in Japan were already low, and other factors (like diagnostic criteria and broader infant care improvements) likely played a major role. By the mid-1980s, Japan’s overall infant mortality rate had fallen dramatically, from about 12–13 per 1,000 in 1970 to ~5 per 1,000 in 1985 (World Bank, 2023). This 60% improvement was driven by advances in neonatal care, infection control, and living standards, which also would have reduced many causes of infant death (including SIDS). In essence, SIDS was a very small contributor to infant mortality in Japan throughout the 1970s–80s, making it challenging to isolate the effect of any single factor like vaccines on its incidence.
Vaccine-Related Mortality and Infant Health Trends
While Japan’s SIDS rates were declining in the long run, vaccine-preventable disease deaths were a countervailing concern during the policy experiment of the late 1970s. The pertussis outbreak of 1977– 1979 demonstrated the cost of lowered immunization: over 100 infant lives were lost to whooping cough in just a few years. Once DTaP was introduced and widely accepted, pertussis deaths again became rare in Japan. By the mid-1980s, Japan had one of the lowest infant mortality rates in the world, and it continued to improve thereafter . Figure 1 summarizes the trends in infant mortality, SIDS, and pertussis outcomes over this period of changing vaccination practices.
Figure 1. Trends in infant health in Japan during changes to DTP immunization. Top: Infant mortality rate (deaths <1 year per 1,000 live births) fell steadily from the 1970s to 1990s . Bottom: Reported pertussis cases and deaths spiked in the late 1970s after infant DTP was halted, then dropped again after acellular DTP was introduced . Japan’s recorded SIDS rate remained low in the 1980s and peaked around the mid-1990s (~0.4/1000) before declining with safe-sleep campaigns. (Source(s): Müller-Nordhorn et al., 2008. International time trends in sudden unexpected infant death. Pediatrics. 122(3): e660–e666.; WHO Mortality Database (cross-validated with national Japanese health statistics) Note: Japan’s SIDS diagnostic practices changed over time, so earlier data may underreport true SIDS cases (often coded as “unknown” or “asphyxia”).; DTP Vaccination Coverage (%) Years: 1970–2000 (estimates held constant after 2000 at >95%), source: WHO/UNICEF Joint Reporting Form (JRF); https://immunizationdata.who.int; National Japanese MHLW reports and coverage data in: Watanabe & Nagai, 2005. Expert Rev Vaccines. 4(2): 173–184. Interpolated for mid-decade years using policy shift data from Cherry et al., 1988.
Despite the temporal correlation between delayed vaccination and low SIDS in the late 1970s, medical research has neither established – nor ruled out – a causal link that vaccines were causing SIDS in the first place. Nevertheless, epidemiological studies – both in Japan and internationally – conclude that DTP immunization does not increase SIDS risk. For example, a 1988 review by Cherry et al. examined Japan’s data and found no persisting excess of “sudden deaths” after vaccination once confounders were “accounted for”; (we would say, “once certain covariates were included in the model”). Likewise, the U.S. Institute of Medicine and other bodies reviewed global evidence and found no causal relationship between DTP and SIDS (Stratton, 2003)). Several large case–control studies were produced that concluded that vaccinated infants have a lower risk of SIDS than unvaccinated infants. A meta-analysis by Vennemann et al. (2007, Vaccine) covering nine studies reported that infants who received routine immunizations had about half the risk of SIDS compared to those who did not (adjusted odds ratio ~0.54; Vennemann et al., 2007).
Researchers suggest this apparently protective association could reflect the “healthy vaccinee” effect (families who vaccinate may practice overall safer care) or even biological benefits of immunization (e.g. prevention of infections that can trigger SIDS). In any case, no robust evidence supports the notion that vaccines were a significant driver of SIDS in Japan.
Confounding Factors and Alternative Explanations
Several confounding factors likely explain why SIDS rates dropped in Japan independently of – or in addition to – vaccine schedule changes:
Changing Sleep Practices: By the 1990s, Japan implemented public health campaigns advising parents to place babies on their back to sleep and avoid known SIDS risk factors. This “Back-to-Sleep” movement (similar to campaigns in Western countries) was credited with halving Japan’s SIDS rate between 1984 and 2004. The timing of the steepest SIDS decline (late 1990s) corresponds to these interventions rather than any vaccine policy change. In fact, SIDS deaths fell in many countries during this period due to safer sleep practices, regardless of vaccination schedules.
Maternal and Environmental Factors: Japanese studies have identified factors like maternal smoking and young maternal age as significant SIDS risks . Japan historically has had lower smoking rates among women and fewer teenage mothers compared to some Western nations, which may have contributed to its relatively low SIDS incidence. These factors are unrelated to vaccination. Recent analysis of Japanese infant deaths found no link with immunization status, instead underscoring maternal health and sleep environment as key determinants.
Diagnostic Criteria and Reporting: How infant deaths get classified can greatly influence “SIDS” statistics. In Japan, only ~40% of sudden unexpected infant deaths are labeled as SIDS; the majority are coded as other causes (e.g. asphyxia of unknown origin). During the 1970s–80s, this conservative diagnostic approach meant some unexplained deaths that might be called SIDS elsewhere were attributed to other causes in Japan. Thus, Japan’s “low SIDS” in that era could partly be a reporting artifact. There is also the point that if an infant wasn’t recently vaccinated (as was the case late-70s), doctors might be even less inclined to classify a death as “SIDS” rather than, say, suffocation or febrile seizure. This makes direct comparisons of SIDS rates before vs. after 1975 difficult.
Overall Infant Health Improvements: The period from 1975 to 1988 saw tremendous improvements in Japan’s maternal-child healthcare system. Expanded prenatal care, improved nutrition, advanced neonatal intensive care, and reduced infectious diseases all contributed to fewer infant deaths across the board. SIDS, being a subset of infant mortality, benefitted from these general gains. For instance, if fewer premature or low-birthweight babies died (factors associated with SIDS risk), the SIDS rate would drop naturally. It’s plausible that Japan’s SIDS decline would have occurred due to these broad improvements, even if the DTP schedule had remained unchanged.
In summary, Japan’s experience in the 1970s–1980s provides a unique historical case. Delaying infant vaccinations was temporally followed by a period of very low reported SIDS, which has fueled speculation about a cause-effect relationship. However, a deeper look reveals that correlation is not causation. The most likely explanation for Japan’s SIDS trends lies in a combination of safer vaccines (reducing vaccine- attributable deaths), better infant care practices, and changes in SIDS risk-factor prevalence – rather than immunizations being a hidden cause of crib death. Japan’s government and pediatric experts have maintained that vaccination is safe and crucial for preventing deadly diseases in infancy. Indeed, as of today Japan achieves over 95% vaccination coverage in infants for diseases like pertussis, and its SIDS rate remains one of the lowest in the world. No rebound of SIDS accompanied the return of early-life DTaP in 1989; if anything, infant survival continued to improve.
Reevaluating the “Back to Sleep” Campaign
A growing body of scholarship, including critiques by Pelligra, Doman, and Leisman, has raised significant concerns about the scientific and ethical foundations of the “Back to Sleep” Campaign (BTSC). Launched in 1994, the BTSC was premised on the claim that placing infants in the supine sleeping position reduces the risk of Sudden Infant Death Syndrome (SIDS). While the campaign has been widely credited with reducing SIDS rates in the United States and elsewhere, closer examination reveals substantial challenges to this conclusion and its associated policy narrative.
One of the most significant concerns is the questionable validity of SIDS mortality statistics. The authors point out, as noted earlier, that there are no internationally consistent diagnostic criteria for SIDS. For example, in Japan, no uniform definition was in place during the 1980s and 1990s. This variability undermines the reliability of global epidemiological data. Even within countries, diagnostic practices have shifted. In Scotland, sudden infant deaths that were categorized as “SIDS” in the 1980s were increasingly labeled as “unknown cause” or “accidental suffocation” by the 1990s. This trend is not unique. In the U.S., an expert panel convened by the NICHD in 1991 revised the criteria for diagnosing SIDS to include comprehensive death scene investigations and medical history reviews—measures that led to increased differentiation between SIDS and other causes of sudden death such as suffocation, metabolic disorders, or infanticide (Willinger et al., 1991). Notably, SIDS rates in the U.S. were already declining before the BTSC began. Between 1989 and 1994, the rate dropped from 1.3 to 1.03 per 1,000 live births, suggesting that factors other than sleep position—such as updated diagnostic protocols and broader public health messaging—may have driven the reduction.
This feeds directly into the diagnostic shift hypothesis. As diagnostic scrutiny increased, deaths once labeled as SIDS were increasingly attributed to other causes. A study by Côté et al. (1999) found that autopsies performed at pediatric pathology centers yielded significantly fewer SIDS diagnoses compared to those conducted at general hospitals or forensic institutes (Côté et al., 1999). In other words, changs in diagnostic tools and classification systems—not necessarily a biological reduction in SIDS risk—may account for much of the observed decline.
The supposed causal relationship between sleep position and SIDS is also called into question. The most often cited study in support of the BTSC’s core claim—Dk et al., 2003—was conducted entirely after the campaign began (Dk et al., 2003). However, this study suffered from low participation rates (50% for SIDS cases, 41% for controls), a small sample size (only 185 cases), and significant risks of recall and social desirability bias. It also failed to fully control for major confounding variables like smoking, co-sleeping, and socioeconomic status. Importantly, no robust follow-up study with higher methodological standards has confirmed its findings. Thus, the central claim that supine sleep reduces SIDS risk remains inadequately supported.
The BTSC, according to its critics, functions more as a massive, unvalidated public health experiment than a proven preventive strategy (Pelligra et al., 2005). Without a confirmed causal mechanism linking prone sleeping to SIDS, and without randomized controlled trials demonstrating benefit, the program rests largely on correlative reasoning. The authors emphasize that, unlike smoking cessation—where the intervention (not smoking) has no known negative side effects—supine sleep is not a neutral intervention. It carries its own risks, which have been largely ignored in public discourse.
Among those risks is a marked increase in positional plagiocephaly, or flat head syndrome, which now affects approximately 1 in 60 U.S. infants (BIGGS, 2003). There are also signs that infants who consistently sleep supine may experience delayed motor development due to reduced opportunities to activate anti-gravity extensor muscles, affecting milestones such as rolling, crawling, and sitting. Some studies also suggest that prone sleeping yields more restful and consolidated sleep—an important factor for neurological development during infancy (Ottolini et al., 1999). These effects are not trivial and appear to be far more common than the number of SIDS cases hypothetically prevented by universal supine sleep enforcement.
The authors further criticize how the campaign’s effectiveness is presented. While proponents often tout a “47% reduction” in SIDS, this dramatic figure translates, in absolute terms, to preventing just one death per 2,127 live births. Meanwhile, the secondary effects—plagiocephaly, developmental delays, and disrupted sleep—impact a much larger portion of the infant population.
Finally, the authors argue that BTSC guidelines, while technically “recommendations,” have become a de facto mandate. Pediatricians feel compelled to adhere to them to avoid liability, and parents are pressured to comply, often against their own instincts or observational judgment. The result is a coercive public health intervention whose scientific basis is, at best, unsettled.
In their conclusion, the authors issue a clear warning: until SIDS is better understood—until causal mechanisms are established and the specific role of sleep position validated—the BTSC should be regarded as investigational. It should not be imposed as standard care. Instead, the decision on sleep position should rest with informed parents who are fully aware of both the potential benefits and risks of supine versus prone sleep.
Do the “Exonerating Studies” Actually Exonerate Vaccines?
First, none of the major studies guarding vaccines from suspicion employed modern protections against model overfit. Logistic regression models were used without penalization, regularization, or external validation. Variables were included based on assumed relevance and their effect on the significance of the effect size of vaccines rather than formally prior understood causal relationships. The studies did not disclose variance inflation factors or tested for collinearity among predictors. This leaves their multivariate estimates vulnerable to distortion, especially given small sample sizes relative to the number of covariates (a low events-per-variable ratio).
Second, many of the variables treated as confounders in these studies are actually co-risk factors, effect modifiers, or mediators. For instance:
● Sleeping position is a direct risk factor for SIDS and likely interacts with vaccine-triggered autonomic vulnerability, yet no study tested for a sleep position × vaccination interaction. New studies designed to test both factors and the interaction term are needed.
● Interactions must similarly be studied for maternal smoking, which modifies immune responses and SIDS risk, but again, its role as a possible interactor, not a confounder, has been ignored.
● Birth weight and prematurity are often on the causal pathway from fetal stress to SIDS, making their inclusion as covariates potentially distorting through collider bias.
The studies used Directed Acyclic Graphs (DAGs) to clarify causal structure or identify proper adjustment sets. They relied on conventional regression without verifying assumptions. The result is an analytical house of cards.
Third, many studies depended on parental recall for vaccine history, or used administrative data without precise immunization timing. Retrospective recall bias, especially under conditions of grief or litigation, undermines internal validity.
Fourth, there is zero consideration for individual susceptibility. No study stratified risk by early developmental signs, genetic markers, or metabolic vulnerability. Signals of risk private to subgroups might be swamped by the Simpson effect. All analyses assume homogeneity of vaccine effect—a premise that is epidemiologically reckless.
Finally, the healthy vaccinee effect looms large. Families who vaccinate on time are typically more health literate, more stable, and more compliant with medical advice. Their children are also less likely to die of all causes. Observing lower SIDS rates among vaccinated infants does not imply protection—it may simply reflect underlying differences in risk unrelated to vaccination.
Biological Vulnerability to Vaccine-Associated SIDS
While population-level studies have not established a causal relationship between vaccination and Sudden Infant Death Syndrome (SIDS), the possibility remains that a small subset of infants may be biologically vulnerable to fatal outcomes following immune stimulation. These cases—rare but potentially avoidable—are statistically invisible in broad observational datasets that average across diverse phenotypes. Recognizing and characterizing this vulnerable subgroup is essential to achieving both precision public health and vaccine safety.
Several biologically plausible mechanisms have been proposed to explain how vaccination, acting as an immune stimulus, might contribute to autonomic destabilization in susceptible infants:
● Mitochondrial dysfunction: Infants with undiagnosed mitochondrial disorders may experience energy failure when faced with the metabolic demands of fever or inflammation, tipping a fragile physiological balance.
● Immune hyperactivation: Excessive or dysregulated cytokine release—particularly interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and other pro-inflammatory mediators—can affect brainstem centers governing respiratory rhythm and cardiovascular control.
● Genetic susceptibility: Mutations in genes like SCN5A (sodium channels), PHOX2B (autonomic development), or cardiac ion channel genes may predispose infants to arrhythmias or defective arousal responses.
● Immature blood–brain barrier (BBB): In neonates, a permeable BBB may allow peripheral cytokines or adjuvant components to interact with central regulatory circuits, particularly during sleep.
● Aluminum induced anemia: Aluminum hydroxide binds to transferrin, outcompeting iron, preventing iron from reaching the bones. Infants may develop anemia as a result. Combined with sleep position, this could be critical and testable factor.
In addition to all of these individual vulnerabilities, sleep state and position are critical contextual factors. The vast majority of SIDS events occur during sleep, typically in the early morning hours when arousal thresholds are high. No study to date has examined whether there is a sleep position × vaccine timing interaction, even though it is plausible that infants vaccinated shortly before entering prone sleep may be at increased risk of failing to arouse from hypoxic or hypercapnic states. This is particularly concerning given that peak SIDS vulnerability overlaps with the timing of primary immunizations at 2 to 4 months of age.
Transient dysregulation of the autonomic nervous system in infants with latent or undiagnosed vulnerabilities is an especially plausible mechanism of SIDS. Vaccination activates pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and other mediators that can cross the blood-brain barrier in neonates and influence brainstem centers responsible for respiration and arousal. In infants with immature or impaired cardiorespiratory integration—particularly during the developmental window of 2 to 4 months—this immune-mediated stress may precipitate apneic episodes, bradycardia, or fatal failure to arouse from sleep. Though rare, this mechanism aligns with observations that some post-vaccine deaths occur during sleep and resemble central apnea phenotypes. The failure of published studies to investigate such mechanistic pathways, or to stratify risk by biomarkers of autonomic maturity or inflammation, represents a profound oversight in SIDS research design. Without such stratification, true susceptible subpopulations remain statistically invisible—averaged out of existence by crude cohort analyses.
Critically, none of the major case-control studies cited to dismiss a vaccine-SIDS link have stratified their analyses by markers of biological risk. There is no disaggregation by sex (male infants are more vulnerable), birth weight, autonomic tone, prior apnea episodes, or genetic predisposition. Nor have these studies incorporated any immune biomarkers, ECG screening, or follow-up assessments to identify subclinical vulnerability.
This failure to model heterogeneity represents a fundamental epistemological error. In a condition as rare and complex as SIDS, the assumption of population homogeneity all but guarantees that signal is lost in noise. If even 1 in 10,000 infants is vulnerable to immune-provoked cardiorespiratory collapse, no cohort study with a thousand subjects and no mechanistic stratification will detect it. What is needed is not simply larger datasets, but smarter design—risk-based subgroup analysis, molecular phenotyping, and longitudinal physiological monitoring.
Until such data are available, it is premature to declare vaccination irrelevant to SIDS. The burden of proof must not rest solely on showing large average effects, but on demonstrating that vulnerable individuals are not harmed in ways that aggregate models cannot detect. Biological vulnerability is not a fringe hypothesis—it is the unstudied frontier of this debate.
Conclusion: The so-called “exonerating studies” do not, in fact, exonerate vaccines. At best, they show that no gross statistical association persists in crude observational data after adjusting for variably chosen covariates. At worst, they mask rare but real vulnerabilities in specific infants who—because of their physiology, environment, or genetics—may be at increased risk of fatal outcomes from early immune challenges. Those cases demand serious, not dismissive, scientific attention. The methodological flaws are not minor technicalities. They are fatal to the conclusions being drawn. The foundation of the “vaccine protects against SIDS” narrative is, scientifically, untenable and is likely an artefact due to arbritary model selection.
Society must grapple with the repeated manipulation of studies, even if intended for the good, because policies based on biased or incomplete data risk attracting blowback in the form of well-earned mistrust by the public, by lawmakers, and debacles such as the public health response to COVID-19.
References
- BIGGS, W. S. (2003). Diagnosis and management of positional head deformity. American Family Physician, 67(9), 1953–1956. https://pubmed.ncbi.nlm.nih.gov/12751657/
- Cherry, J. D., Brunell, P. A., Golden, G. S., & Karzon, D. T. (1988). Report of the Task Force on Pertussis and Pertussis Immunization—1988. Pediatrics, 81(6), 933–984. https://doi.org/10.1542/peds.81.6.933
- Côté, A., Russo, P., & Michaud, J. (1999). Sudden unexpected deaths in infancy: What are the causes? The Journal of Pediatrics, 135(4), 437–443. https://doi.org/10.1016/s0022-3476(99)70165-4
- Dk, L., Db, P., M, W., R, M., R, O., H, V., & Hj, H. (2003). Infant sleeping position and the risk of sudden infant death syndrome in California, 1997-2000 – PubMed. American Journal of Epidemiology, 157(5). https://doi.org/10.1093/aje/kwf226
- Kuno‐Sakai, H., & Kimura, M. (2004). Safety and efficacy of acellular pertussis vaccine in Japan, evaluated by 23 years of its use for routine immunization. Pediatrics International, 46(6), 650–655. https://doi.org/10.1111/j.1442-200x.2004.01970.x
- Müller-Nordhorn, J., Schneider, A., Grittner, U., Neumann, K., Keil, T., Willich, S. N., & Binting, S. (2020). International time trends in sudden unexpected infant death, 1969–2012. BMC Pediatrics, 20(1). https://doi.org/10.1186/s12887-020-02271-x
- Ottolini, M. C., Davis, B. E., Patel, K., Sachs, H. C., Gershon, N. B., & Moon, R. Y. (1999). Prone infant sleeping despite the “back to sleep” campaign. Archives of Pediatrics & Adolescent Medicine, 153(5). https://doi.org/10.1001/archpedi.153.5.512
- Pelligra, R., Doman, G., & Leisman, G. (2005). A reassessment of the SIDS back to sleep campaign. The Scientific World JOURNAL, 5, 550–557. https://doi.org/10.1100/tsw.2005.71
- Sawaguchi, T., Fujita, T., Sawaguchi, A., & Nishida, H. (2000). The epidemiological study on registered cases of sudden infant death syndrome (SIDS) in Tokyo: Examination of the effect of autopsy on diagnosis of SIDS and the mortality statistics in Japan. Forensic Science International, 109(1), 65–74. https://doi.org/10.1016/s0379-0738(99)00218-2
- Stratton, K. R. (2003). Immunization safety review: Vaccinations and sudden unexpected death in infancy. National Academy Press. https://www.ncbi.nlm.nih.gov/books/NBK221465/
- Vennemann, M. M. T., Höffgen, M., Bajanowski, T., Hense, H.-W., & Mitchell, E. A. (2007). Do immunisations reduce the risk for SIDS? A meta-analysis. Vaccine, 25(26), 4875–4879. https://doi.org/10.1016/j.vaccine.2007.02.077
- Warfel, J. M., Zimmerman, L. I., & Merkel, T. J. (2013). Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proceedings of the National Academy of Sciences, 111(2), 787–792. https://doi.org/10.1073/pnas.1314688110
- Watanabe, M., & Nagai, M. (2005). Acellular pertussis vaccines in Japan: Past, present and future. Expert Review of Vaccines, 4(2), 173–184. https://doi.org/10.1586/14760584.4.2.173
- Willinger, M., James, L. S., & Catz, C. (1991). Defining the sudden infant death syndrome (sids): Deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatric Pathology, 11(5), 677–684. https://doi.org/10.3109/15513819109065465
- World Bank. (2023). World Bank open data. World Bank Open Data. https://data.worldbank.org/indicator/SH.DYN.MORT?locations=JP
**********************************************************************************************************************